Jag träffade Cecilia Fürst i förrgår och vi pratade om Covid-19. Cecilia som ju bl.a. är expert på genetik tog upp de stora genetiska skillnaderna i hur hårt vi drabbas av SARS-CoV-2, det nya Coronaviruset.
Som jag skrivit om i tidigare inlägg så är proteinet ACE2 det här virusets inkörsport i våra celler (mer via https://4health.se/?s=ACE2 och https://4health.se/?s=corona )
Det finns olika genetiska varianter för ACE2-receptorer. Och det verkar som om dessa skillnader varierar mycket mellan befolkningar, vilket gör att personer med påbrå från tex mellanöstern har betydligt högre risk att drabbas hårt av Covid-19 jämfört med oss europeiska nordbor. Även sydeuropéer kan ha “värre” genetiska anlag än oss i norra Europa i den här bemärkelsen.
Även skillnaden mellan män och kvinnor kan bero på detta (att män generellt drabbas hårdare av Covid-19).
Något kontroversiellt, men troligen en förklaring till att personer med utländsk härkomst drabbats värre även i Sverige. Det beror alltså troligen inte bara på att man umgås mer över generationsgränserna i vissa kulturer.
Se citat på ämnet/studier från Nature nedan och där finns även en länk till en sida som är än mer uppdaterad och där man samlar genetiska data gällande Covid-19 och ACE2
Mer via https://4health.se/?s=corona
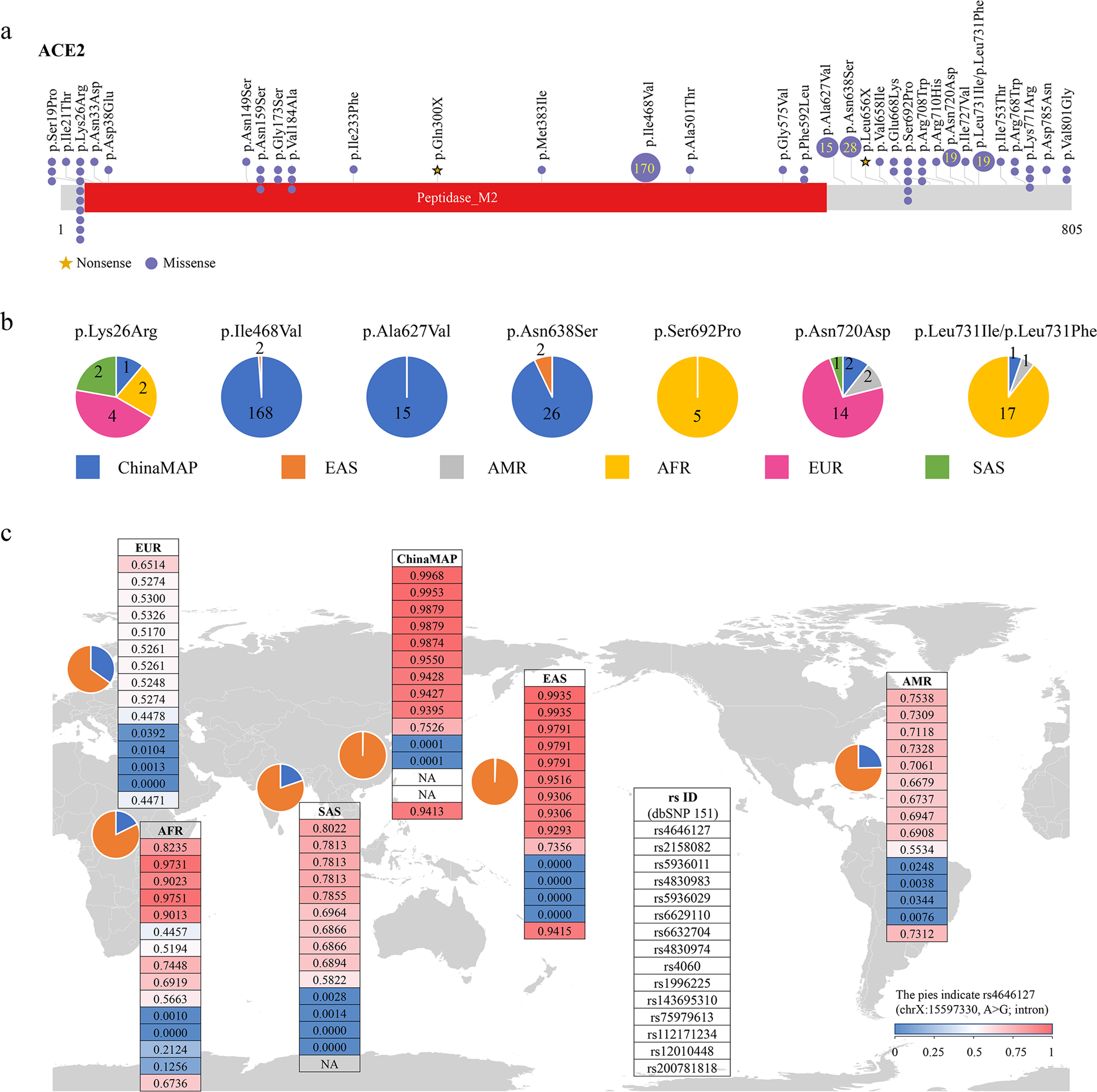
Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations
The ACE2 gene encodes the angiotensin-converting enzyme-2, which has been proved to be the receptor for both the SARS-coronavirus (SARS-CoV) and the human respiratory coronavirus NL63. Recent studies and analyses indicate that ACE2 could be the host receptor for the novel coronavirus 2019-nCoV/SARS-CoV-21,2. Previous studies demonstrated the positive correlation of ACE2 expression and the infection of SARS-CoV in vitro3,4. A number of ACE2 variants could reduce the association between ACE2 and S-protein in SARS-CoV or NL635. Therefore, the expression level and expression pattern of human ACE2 in different tissues might be critical for the susceptibility, symptoms, and outcome of 2019-nCoV/SARS-CoV-2 infection. A recent single-cell RNA-sequencing (RNA-seq) analysis indicated that Asian males may have higher expression of ACE26. Currently, the clinical reports of 2019-nCoV/SARS-CoV-2 infection from non-Asian populations for comparison are very limited. A study from Munich reported four German cases, all of which showed mild clinical symptoms without severe illness7. However, the genetic basis of ACE2 expression and function in different populations is still largely unknown. Therefore, genetic analysis of expression quantitative trait loci (eQTLs)8 and potential functional coding variants in ACE2 among populations are required for further epidemiological investigations of 2019-nCoV/SARS-CoV-2 spreading in East Asian (EAS) and other populations.
To systematically investigate the candidate functional coding variants in ACE2 and the allele frequency (AF) differences between populations, we analyzed all the 1700 variants (Supplementary Table S1) in ACE2 gene region from the ChinaMAP (China Metabolic Analytics Project, under reviewing) and 1KGP (1000 Genomes Project)9 databases. The AFs of 62 variants located in the coding regions of ACE2 in ChinaMAP, 1KGP, and other large-scale genome databases were summarized (Supplementary Table S2). All of the 32 variants potentially affecting the amino acid sequence of ACE2 in databases were shown (Fig. 1a). Previous study showed that the residues near lysine 31, and tyrosine 41, 82–84, and 353–357 in human ACE2 were important for the binding of S-protein in coronavirus5. The mutations in these residues were not found in different populations in our study. Only a singleton truncating variant of ACE2 (Gln300X) was identified in the ChinaMAP (Fig. 1a). These data suggested that there was a lack of natural resistant mutations for coronavirus S-protein binding in populations. The effects of low-frequency missense variants in populations for S-protein binding could be further investigated. The distributions of seven hotspot variants (Lys26Arg, Ile468Val, Ala627Val, Asn638Ser, Ser692Pro, Asn720Asp, and Leu731Ile/Leu731Phe) in different populations were shown (Fig. 1b). Six low-frequency loci (rs200180615, rs140473595, rs199951323, rs147311723, rs149039346, and rs73635825) were found to be specific in 1KGP database, the AFs of which were also low in the gnomAD and TopMed10database. Only two of these six variants (rs200180615 and rs140473595) could be found in CHB (Han Chinese in Beijing) population with the AF < 0.01. Interestingly, the SNP rs2285666 with the highest AF in the 62 variants exhibited much higher AF in the ChinaMAP (0.556) and CHS (Han Chinese South, 0.557) populations compared to others (AMR, Ad Mixed American, 0.336; AFR, African, 0.2114; EUR, European, 0.235). In addition, the homozygous mutation rate in males (0.550) was much higher than females (0.310) in the Chinese population (Supplementary Table S2). Taken together, the differences in AFs of ACE2 coding variants among different populations suggested that the diverse genetic basis might affect ACE2 functions among populations.
To analyze the distribution of eQTLs for ACE2, we used the Genotype Tissue Expression (GTEx) database (https://www.gtexportal.org/home/datasets). We found 15 unique eQTL variants (14 SNPs and 1 INDELs) for ACE2 with q value ≤ 0.05 in 20 tissues from the GTEx database (rs112171234, rs12010448, rs143695310, rs1996225, rs200781818, rs2158082, rs4060, rs4646127, rs4830974, rs4830983, rs5936011, rs5936029, rs6629110, rs6632704, and rs75979613). The AFs of the 15 eQTL variants were compared among different populations. Notably, our results showed most of the 15 eQTL variants had much higher AFs in the ChinaMAP dataset and EAS populations compared to European populations (Fig. 1c and Supplementary Table S3). The AFs of the top 6 common variants (rs4646127, rs2158082, rs5936011, rs6629110, rs4830983, and rs5936029) were higher than 95% in EAS populations, whereas the AFs of these variants in European populations were much lower (52%–65%). All of the 11 common variants (AF > 0.05) and 1 rare variant (rs143695310) in the 15 eQTLs are associated with high expression of ACE2 in tissues (Supplementary Table S3). For instance, the eQTL variant rs4646127 (log allelic fold change = 0.314), which locates in the intron of ACE2 gene, has the highest AFs in both of the ChinaMAP (0.997) and EAS (0.994) populations. Comparatively, the AFs of rs4646127 in EUR (0.651) and AMR (0.754) populations are much lower. These findings suggested the genotypes of ACE2 gene polymorphism may be associated higher expression levels of ACE2 in EAS population.
Recent reports of the ACE2 expression analysis in lung tissues from Asian and Caucasian populations are still controversial. The single-cell RNA-seq analysis reported that the Asian donor had much higher ACE2 expression cell ratio than white and African-American donors6. In contrast, the ACE2 expression analysis using the RNA-seq and microarray datasets from control lung tissues indicated there were no significant differences between Asian and Caucasian, or male and female11. The ACE2-expressing cells are a very small part of cells in lung tissues6. The sample size and the purity of ACE2-positive cells in the selected samples would influence the conclusions. Our analysis showed the differences in distribution and AFs of eQTLs for ACE2 in different populations, indicating the diversity of ACE2 expression pattern in populations (Supplementary Table S3). Large-scale and multiple tissue-level analysis of single-cell RNA-seq would be more accurate for the expression analysis of ACE2 in different populations. In addition, our data showed the moderate difference in AFs of eQTLs between South Asian and EAS, which suggests the potential difference of ACE2 expression in different populations and ethnics in Asia (Fig. 1c).
In summary, we systematically analyzed coding-region variants in ACE2and the eQTL variants, which may affect the expression of ACE2 using the GTEx database to compare the genomic characteristics of ACE2among different populations. Our findings indicated that no direct evidence was identified genetically supporting the existence of coronavirus S-protein binding-resistant ACE2 mutants in different populations (Fig. 1a). The data of variant distribution and AFs may contribute to the further investigations of ACE2, including its roles in acute lung injury and lung function12. The East Asian populations have much higher AFs in the eQTL variants associated with higher ACE2 expression in tissues (Fig. 1c), which may suggest different susceptibility or response to 2019-nCoV/SARS-CoV-2 from different populations under the similar conditions.
https://www.nature.com/articles/s41421-020-0147-1
Websida som samlar genetiska data på detta ämne: https://www.covid19hg.org

Jag känner viss tvekan. Om man läser tex Karin Boijs böcker om genetik så står det klart att väldigt många av skandinaver och nord-europeer har mycket genetik från mellanöstern, dessa gener vandrade in med jordbrukare för många tusen år sedan. Grovt kan man säga att den från far till son ärvda y-kromosomen finns i bara 3 varianter i den här regionen (se Boijs) varav den näst största är den från dessa jordbrukare. På den kvinnliga sidan finns det några fler varianter av x-kromosomen men grovt kan man säga att det är 5 varianter och någon några från mellanöstern. Eftersom de flesta av oss är blandningar av dessa så kan man nog säga att vi sedan mycket länge är till stor del genetiskt mellanöstriska :-).
Alla har väl genetik därifrån om vi ska hårddra det. Här handlar det nog mest om uttrycken för ACE-receptorerna och där tycks det skilja sig åt.