Det är vid det här laget väl känt att män generellt drabbas mer och hårdare än kvinnor av det nya coronaviruset. Fler män blir svårt sjuka, fler män vårdas på IVA och fler män dör med covid-19. I Sverige har 800 fler män dött i covid-19 än kvinnor, trots att fler kvinnor testat positivt för sjukdomen. Om man tittar globalt så är risken för att dö med covid-19 ungefär 1,7 gånger högre för män än för kvinnor. I en ny artikel i Science sammanfattar forskare den kunskap och de teorier som finns hittills om vad det här kan bero på – och många teorier handlar om att det är skillnader i kvinnors och mäns immunförsvar som ligger bakom.
Jag har skrivit om det i tidigare inlägg, bl.a. en del här https://4health.se/hur-hart-vi-drabbas-av-corona-beror-pa-genetiska-skillnader-i-olika-varldsdelar-befolkningar och här https://4health.se/?s=män+drabbas . Läs gärna också om skillnaderna mellan kvinnors och mäns immunsystem: https://4health.se/skillnader-mellan-det-manliga-och-det-kvinnliga-immunsystemet-forklarar-skillnader-i-sjukdomar
Det finns flera skillnader mellan mäns och kvinnors immunförsvar. En sak är att de åldras olika. Hos båda könen försvagas immunförsvaret med åldern, framförallt efter 60 år. Men den försvagningen är kraftigare hos män och sker normalt 5-6 år tidigare än hos kvinnor. Det gör att män, särskilt då äldre män, har svårare att kontrollera virusinfektioner.
Men män har också generellt ett sämre immunförsvar än kvinnor och i alla åldersgrupper över 30 år ser man att män drabbas hårdare och är överrepresenterade när det gäller döda med covid-19. Till och med mer än vid många andra infektioner.
Dessutom finns viktiga immunrelaterade gener på X-kromosomen.
I möss har man sett att receptorn som SARS-Cov2-viruset binder till (ACE2) kan regleras av könshormonet östrogen. Det skulle kunna förklara ytterligare en skillnad i hur man reagerar på infektionen.
Forskarna reflekterar också kring andra kända skillnader mellan män och kvinnors immunsystem, såsom “females generally mount a more robust immune response to vaccines, such as influenza vaccines. However, the heightened immune responses in females can also lead to detrimental immunopathology in infections” Jag reflekterar här över kvinnor som utvecklar långtidssymtom efter covid, samt utvecklar autoimmuna sjukdomar efter infektion generellt.
Läs mer om skillnaderna i immunsystemet i nedan text ur artikeln.
Mer via https://4health.se/?s=corona
Ur artikeln:
“the risk of death in males is ∼1.7 times higher than in females (1). Aging is strongly associated with higher risk of death in both sexes, but at all ages above 30 years, males have a significantly higher mortality risk, rendering older males the most vulnerable group (1). Sex differences are intertwined with differences in gender roles socially and with behavioral factors, which also influence COVID-19 incidence and outcomes. However, there are also possible biological mechanisms of male sex bias that affect the severity of COVID-19, particularly with respect to immune responses.”
“male sex is more often associated with lower immune responses and higher susceptibility and/or vulnerability to infections in animals. This is generally also the case in humans (2): Male patients have higher viral loads for hepatitis B virus (HBV) and HIV (2). Conversely, females generally mount a more robust immune response to vaccines, such as influenza vaccines. However, the heightened immune responses in females can also lead to detrimental immunopathology in infections”
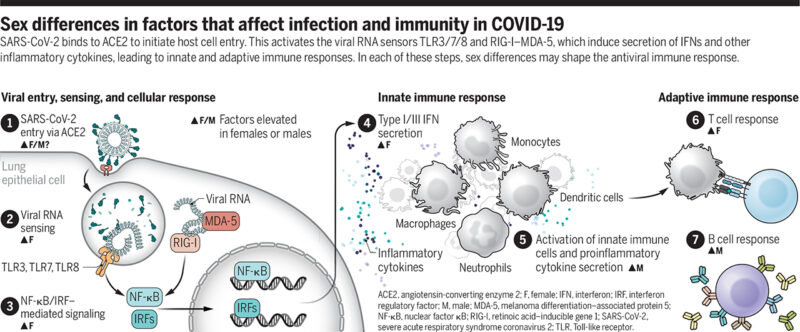
“The physiological response to virus infection is initiated when virus replication is detected by pattern recognition receptors. This leads to two antiviral programs by the infected cells: (i) cellular antiviral defense programs mediated by type I and type III interferons (IFNs) to limit viral replication and spread, and (ii) cytokine and chemokine production to recruit and coordinate immune cells, such as monocytes and neutrophils that can phagocytose and eliminate infected cells. Notably, COVID-19 is characterized by strong innate immune cytokine and chemokine responses despite the disproportionately low antiviral defense signature mediated by IFNs (3). Patients with severe COVID-19 exhibit high serum concentrations of proinflammatory cytokines and chemokines, with particularly high concentrations of interleukin-6 (IL-6) and the inflammasome-associated cytokines IL-1β and IL-18 (4). Exacerbated systemic inflammation is associated with extensive pulmonary pathology, including massive infiltration of monocytes and neutrophils. Increased neutrophil count is associated with poor clinical outcome (5). The robust induction of these inflammatory cytokines and cells may be a compensatory response to the ability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to evade IFN responses, which necessitates the engagement of IFN-independent defense mechanisms (6).
The plasma concentrations of several key innate immune cytokines and chemokines, such as IL-8 and IL-18, are increased in male patients compared with female patients in the early phase of COVID-19. Conversely, female patients have higher plasma concentrations of type I IFN (IFNα) over the disease course (7). Notably, autoantibodies that inhibit type I IFN signaling have been reported in a subset of severely ill patients, the majority (94%) of whom were older males (8). By contrast, T cell activation at the early phase of SARS-CoV-2 infection is robust even in older female patients, whereas male patients have a significant decline with age. Male patients with poor T cell activation at the early phase of disease onset have worse COVID-19 outcomes, whereas no such difference is observed in female patients (7).
What could be the potential underlying mechanism of this sex dimorphism in immune response? One culprit is the sex chromosomes: A substantial number of important immune-related genes are encoded on the X chromosome. Although one of the two copies of the X chromosome in females is usually epigenetically silenced [X chromosome inactivation (XCI)], some critical immune-related genes, including Toll-like receptor 7 [TLR7, whose viral RNA sensing elicits strong type I IFN production in its major producer, plasmacytoid dendritic cells (pDCs)], can escape from XCI in some proportion of cells. This makes the population “mosaic” for biallelic expression, which leads to higher gross expression of some immune-related genes in females (9). Human pDCs have also been reported to have higher expression of interferon regulatory factor 5 (IRF5) in females. Higher expression of these genes leads to more robust type I IFN responses in females, and this is one of the potential mechanisms involved in females’ enhanced protection against viral infections, including COVID-19 (1, 2).
Sex has a major impact on immune cell transcriptomes; immune cells or even the immune system are differentially affected by aging, depending on sex. Aging induces a decline in the proportion of naïve T cells that is more prominent in males, and B cells decline after age 65 only in males (10). Males have abrupt and drastic changes in the epigenetic landscape of their immune cells between ages 62 and 64, and subsequently males exhibit an accelerated immunosenescence phenotype that is characterized by enhanced innate proinflammatory gene expression and lower gene expression related to adaptive immunity, which could potentially predispose older males to hyperinflammation and poor adaptive immune responses.
By contrast, major changes in the epigenetic landscape of immune cells occur in females 5 to 6 years later than in males, with this gap largely corresponding to the life span differences between sexes (10). It is notable that females generally mount more pronounced cytokine responses in viral infections, although this is not the case with COVID-19. Instead, males have higher plasma concentrations of innate proinflammatory cytokines such as IL-8 and IL-18 (7). This could be because patients who have severe disease are generally of older age, and the background transcriptomic and epigenetic sex differences in immune cells in these older individuals are possibly amplified and more explicitly manifested in the context of SARS-CoV-2 infection (see the figure).
The other important biological factors are sex hormones. In a mouse model of SARS-CoV infection, higher mortality in male mice was observed and was attributed to the protective roles of the female sex hormone estrogen (1). Studies using various cell types and animal models have shown that the expression of angiotensin-converting enzyme 2 (ACE2), the host cell entry receptor for SARS-CoV-2, is modulated by estrogen (1). This likely occurs through transcriptional regulation by estrogen receptor signaling, although the detailed molecular mechanisms are yet to be identified and the consequences of the type of ACE2 modulation (up- or down-regulation) could be cell type– and context-dependent. Estrogen receptors are widely expressed by many cell types, including immune cells, and estrogen is a critical regulator of gene expression and functions in innate immune cells, including monocytes, macrophages, and dendritic cells, as well as in lymphocytes such as T helper 1/2 (TH1/2) cells, regulatory T cells (Tregs), and B cells. One of the major forms of estrogen, estradiol, has been shown to dampen the production of excessive innate inflammatory cytokines by monocytes and macrophages (1). Menopause is an independent risk factor for female patients, and concentrations of estradiol and anti-Müllerian hormone, which is produced by cells in developing follicles and is an endocrine marker for oocytes remaining in the ovaries (ovarian reserve), are inversely correlated with COVID-19 severity (11). Conversely, androgen-deprivation therapies for prostate cancer have been reported to reduce the risk for SARS-CoV-2 infection (12). Like estrogen receptors, androgen receptors are expressed widely among immune cells, regulating the transcription of various genes, and the outcome of their signaling has been shown to be generally immunosuppressive (1).
Despite the emerging understanding of sex differences of immune responses in COVID-19, many questions remain. Sex is not binary, and little is known regarding immune responses to viral infections, including COVID-19, in individuals with disorders of sex development (DSD) or transgender individuals. DSD describes congenital conditions in which the development of chromosomal, gonadal, and anatomical sex is atypical. For example, Klinefelter syndrome (also known as 47, XXY) results in phenotypes such as gynecomastia (enlarged breast tissue in males) and small testes with hypogonadism. This condition accompanies a series of comorbidities, in particular a high frequency of autoimmune diseases, such as systemic lupus erythematosus (SLE). The prevalence of SLE among Klinefelter syndrome patients is higher by a factor of 14 than among 46, XY males; this is comparable to its prevalence among 46, XX females, which suggests a gene dosage effect of the X chromosome (13). Additionally, transgender is a collective term encompassing those whose gender identities or gender roles differ from those typically associated with the sex they were assigned at birth. Little is known about the immune responses in these individuals, some of whom are undergoing gender reassignment hormone therapies. It is possible that DSD and transgender individuals mount distinct immune responses to viral infection in general and also in COVID-19.
Sex differences in immunity also have implications for responses to SARS-CoV-2 vaccination or reinfection. Analysis of convalescent plasma showed that male sex, older age, and hospitalization for COVID-19 were associated with greater SARS-CoV-2 antibody titers (14). This could be related to increased disease severity in this demographic of patients, with increased viral load driving more B cell activation and antibody production. Alternatively, higher amounts of antiviral antibodies could be due to compensatory increases in antibody production due to suboptimal qualities of antibodies generated in older males, which are unable to neutralize the virus effectively. Antibodies with suboptimal neutralizing ability have the capacity to promote viral invasion into host cells such as macrophages [called antibody-dependent enhancement (ADE)]. However, there has been no clear evidence of ADE in COVID-19. With other viral infections, fatal diseases in dengue virus infection (dengue hemorrhagic fever and dengue shock syndrome) have been associated with ADE. Upon secondary infection with a virus of a serotype different from that of the primary infection, the fatalities from these severe diseases were primarily seen in adult females despite the male dominance in dengue hemorrhagic fever prevalence (15). The mechanism remains unknown but may be due to heightened inflammation in females, leading to capillary permeability.
It is important that studies of COVID-19 patients report results in a sex-disaggregated manner, not only to elucidate differential disease pathogenesis, but also to enable a deeper understanding of this disease and the eventual development of better treatment and preventive strategies. It should be standard practice to collect and report sex-disaggregated data for this and for all infectious disease and vaccine studies in the future.”
https://science.sciencemag.org/content/371/6527/347
https://www.dn.se/vetenskap/forskare-nya-teorier-om-varfor-man-drabbas-hardare-av-covid-19/

Män dör men kvinnor drabbas oftare av långtidscovid.
“Emma Borgström, 39, har inte kunnat återgå till ett normalt liv sedan hon insjuknade i covid-19 i mars. Hon är en av de långtidssjuka unga högpresterande kvinnor som tycks bli allt fler, enligt covidmottagningarna.
– Jag var frisk och cyklade flera mil till jobbet tidigare, i dag orkar jag knappt stå upp, säger hon.”
https://www.dn.se/ekonomi/emma-lider-av-langtids-covid-kanns-som-att-huvudet-ska-explodera/
Det verkar vara väldigt många som drabbas av långtidscovid (även män)
Novus har mer statistik om långtidscovid .
För dom under 65 år kan nog långtidscovid vara ett större problem än risken att dö.
Det är dom gamla som främst dör och syns i statistiken när vi fokuserar på överdödlighet.
Precis så. Ofta kvinnor med underliggande utmattning eller autoimmuna sjukdomar
Skrivit en del om detta med långtids-covid i tidigare inlägg.
Är det verkligen underliggande sjukdomar på alla?
Det verkar vara fult friska yngre kvinnor som drabbas av långtidscovid.
från DN
“Det är främst kvinnor i ung ålder mellan 25 till 45. Det klassiska är att de har varit väldigt friska och högpresterande mitt i livet, säger Judith Bruchfeld som är överläkare på Karolinska universitetssjukhusets covidklinik.”
Det är inte stressymtom möjligen kan stress gjort personerna mer känsliga för infektionen, men det är något allvarligt som har hänt i deras kroppar och ingen verkar riktigt veta vad som är fel, hemskt är det.
Det ser ut som att du tillhör den riskgruppen ung frisk aktiv.
Har du haft corronan?
Det ska även finnas en del barn som är drabbade, ju yngre ju mindre underliggande sjukdomar finns.
https://4health.se/forklaringen-till-varfor-en-del-friska-halsosamma-personer-blir-svart-sjuka-i-covid-19-autoimmunitet-och-eller-genetik
Underliggande sjukdomar tog du upp i länken men gener som vanligtvis inte orsakar besvär ska det kallas underliggande sjukdom?
neandertalargener är det underliggande sjukdom?
Antikroppar om dom fanns förre virusinfektionen så är det underliggande sjukdom.
Det du tog upp borde vara saker som kanske inte alltid ger symtom.
Det var bra saker du tog upp men hur ska det tolkas.
Det som jag tänkte på var underliggande sjukdomar som ger symtom typ metabola syndromet eller vanliga folksjukdomar.
Diagnoser ställda av läkare.
Det verkade kvinnorna inte ha
Kvinnorna kände sig friska före coronnan tolkar jag det som.
precis – sjukdom eller genetik syftade jag på
Hej, det verkar som att det “nya” syndromet “långtidseffekter av Covid-19”, inte är något nytt utan en kronisk sjukdom som borde vara välkänd bland läkare, forskare, myndigheter och vårdpersonal. Så är dock inte fallet.
Denna hemska sjukdom heter ME/CFS. Den vanligaste orsaken eller åtminstone utlösande faktorn är en infektion, oftast viral infektion.
Tyvärr är det nog ME/CFS som många beskriver efter Covid-19, om det är så att det inte har blivit bra efter 6 månader och de har de typiska symptomen.
Jag har knappt hört detta nämnas i svenska medier, förutom av professor Gottfries.
ME/CFS kan drabba människor i alla åldrar även barn, men det är vanligare att det debuterar mellan 20 och 50 år. De flesta har upplevt sig som helt friska innan och även haft klockrena hälsotester samt levt ett hälsosamt liv.
ME/CFS är nästintill okänd av läkare trots att man känt till den sedan 1950-talet, och den är inte speciellt ovanlig heller. Det finns en hel del forskning publicerad, det finns privata specialistläkare och en stor mängd sjuka människor. Det verkar inte ingå i läkarutbildningen.
Jag instämmer i mycket av det du skriver. Min erfarenhet i det här är att de kvinnor som främst drabbas av långtids-covid är de som har utmattning / kronisk stress i grunden. Detta får dem att tippa över