
En genomgång av forskningen på användning av vitamin C vid Covid-19 visar att intravenös användning av C-vitamin minskar allvarlighetsgrad och förbättrar överlevnad hos personer med sepsis och lunginflammation (dvs sjukdomar som det nya coronaviruset ofta leder till i form av allvarlig Covid-19) “Administration of intravenous vitamin C to patients with pneumonia and sepsis appears to decrease the severity of the disease and potentially improve survival rate”
Den visar också att patienter med covid-19 ofta har utarmat sina nivåer av C-vitamin.
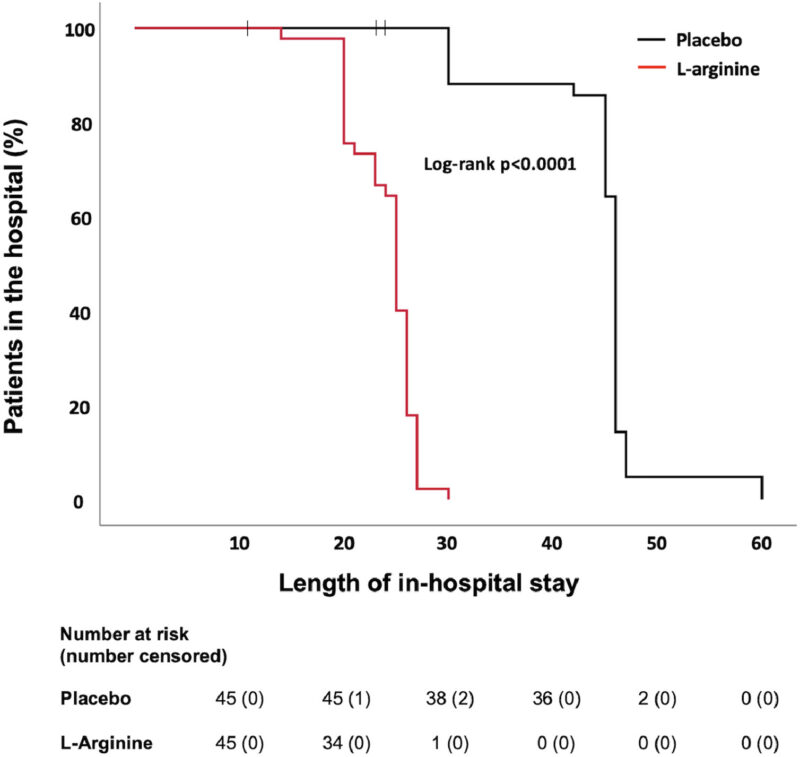
12 studier på C-vitamin i Covid-19 har publicerats, varav 5 är av högsta kvalitet (RCT-studier). Intravenös användning av C-vitamin minskar inflammation och ökar syresättning enligt dessa studier. Det minskar också längden av sjukhusvistelse och minskar dödlighet.
Oral användning av C-vitamin verkar kunna förbättra tillfrisknandet även i mindre allvarliga fall av Covid.
Inga biverkningar har rapporterats.
Ur studien (eller review:n):
Review Vitamin C Intervention for Critical COVID-19: A Pragmatic Review of the Current Level of Evidence
Abstract
Severe respiratory infections are characterized by elevated inflammation and generation of reactive oxygen species (ROS) which may lead to a decrease in antioxidants such as vitamin C and a higher requirement for the vitamin. Administration of intravenous vitamin C to patients with pneumonia and sepsis appears to decrease the severity of the disease and potentially improve survival rate. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes pneumonia, sepsis and acute respiratory distress syndrome (ARDS) in severe cases, and is referred to as coronavirus disease 2019 (COVID-19). Patients with COVID-19 infection also appear to have depleted vitamin C status and require additional supplementation of vitamin C during the acute phase of the disease. To date there have been 12 vitamin C and COVID-19 trials published, including five randomised controlled trials (RCTs) and seven retrospective cohort studies. The current level of evidence from the RCTs suggests that intravenous vitamin C intervention may improve oxygenation parameters, reduce inflammatory markers, decrease days in hospital and reduce mortality, particularly in the more severely ill patients. High doses of oral vitamin C supplementation may also improve the rate of recovery in less severe cases. No adverse events have been reported in published vitamin C clinical trials in COVID-19 patients. Upcoming findings from larger RCTs will provide additional evidence on vitamin supplementation in COVID-19 patientsConclusions
Observational studies have indicated that patients with COVID-19 have high rates of hypovitaminosis C and vitamin C deficiency, which are comparable to patients with sepsis and septic shock [7]. Previous studies of high-dose IVC administration in patients with sepsis and ARDS have shown reduced duration of ICU and hospital stay as well as decreased mortality [27,63]. Based on the evidence from preliminary RCTs and retrospective cohort studies, vitamin C appears to also support positive outcomes in COVID-19 in both inpatient and outpatient settings, leading to a beneficial effect in patients with moderate symptoms, as well as patients with pneumonia, sepsis and ARDS. Intervention with IVC resulted in improved oxygenation and pulmonary function, reduced inflammatory markers and temperature, decreased days in ICU and hospital, and decreased mortality, particularly in the more severely ill patients. Oral supplementation in mild to moderate cases also increased the rate of recovery when taken in high-doses.Overall, the intervention studies to date have a number of limitations such as small sample sizes and early termination of some studies, differences in vitamin C dose and duration and lack of optimisation of these, lack of placebo controls, and no pre-and post-intervention plasma vitamin C concentrations. However, living meta-analysis of the studies as they are published is supporting the premise that high-dose IVC can improve the complications associated with COVID-19 and potentially decrease mortality [64]. Notably, there have been no adverse events reported in the published trials to date.






 Om man vill djupdyka och få kunskap och förståelse kring sin blodsockerreglering och insulinkänslighet så är en glukosmätare som kontinuerligt mäter glukosnivåerna ett väldigt fint verktyg. Det kallas CGM – Continuous Glucose Monitoring.
Om man vill djupdyka och få kunskap och förståelse kring sin blodsockerreglering och insulinkänslighet så är en glukosmätare som kontinuerligt mäter glukosnivåerna ett väldigt fint verktyg. Det kallas CGM – Continuous Glucose Monitoring.  CGM-sensorn fästs på baksidan av armen och en tunn nål mäter glukosnivån en gång per minut i den vätska som finns mellan cellerna strax under huden. Man synkar sensorn via en app i telefonen och sensorn fungerar i 14 dagar och man har alltså på den när man duschar, tränar, sover mm och lägger in information i appen kring vad som sker under dagarna – t ex när och vad man äter.
CGM-sensorn fästs på baksidan av armen och en tunn nål mäter glukosnivån en gång per minut i den vätska som finns mellan cellerna strax under huden. Man synkar sensorn via en app i telefonen och sensorn fungerar i 14 dagar och man har alltså på den när man duschar, tränar, sover mm och lägger in information i appen kring vad som sker under dagarna – t ex när och vad man äter. 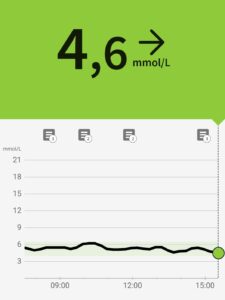 Med en CGM på armen får man i stort sett en realtids-feedback i mobilen på hur blodsockret påverkas av den kost man äter, hur ofta man äter, när man äter, hur och när man sover, tränar och stressar. Och det ger därmed ett väldigt fint underlag för att fatta beslut kring nya val och förändring. Som civilingenjör blir man ju tårögd av glädje att denna typ av wearable finns.
Med en CGM på armen får man i stort sett en realtids-feedback i mobilen på hur blodsockret påverkas av den kost man äter, hur ofta man äter, när man äter, hur och när man sover, tränar och stressar. Och det ger därmed ett väldigt fint underlag för att fatta beslut kring nya val och förändring. Som civilingenjör blir man ju tårögd av glädje att denna typ av wearable finns. 

Senaste kommentarer